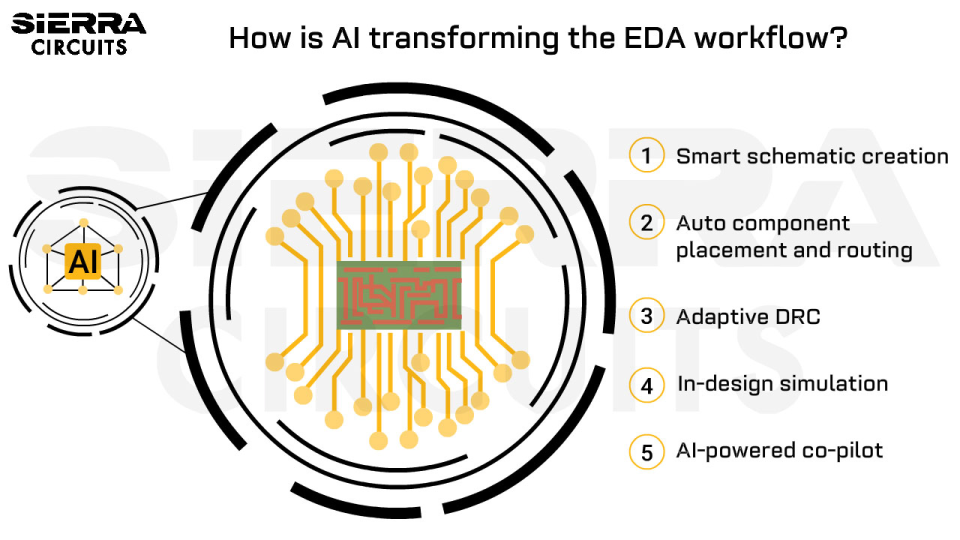
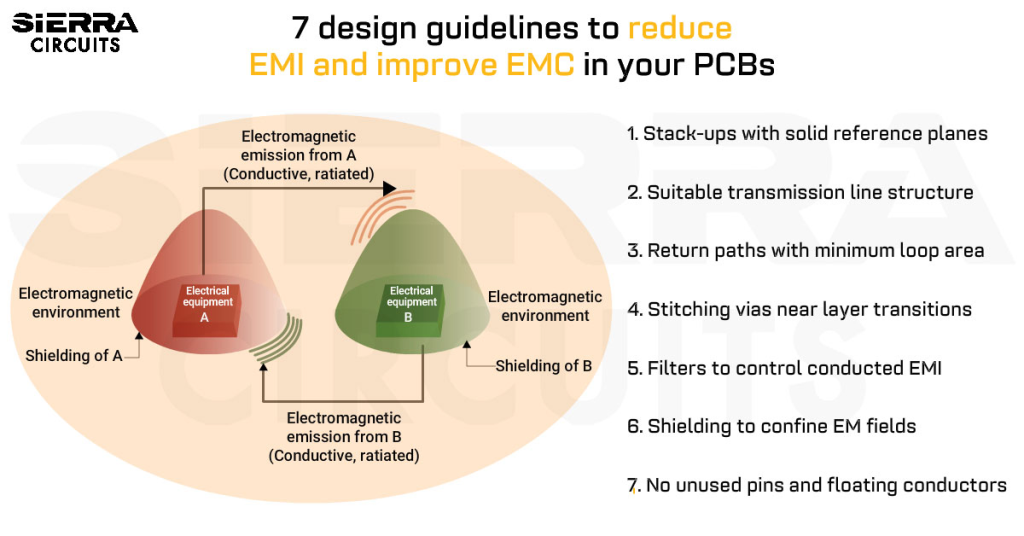
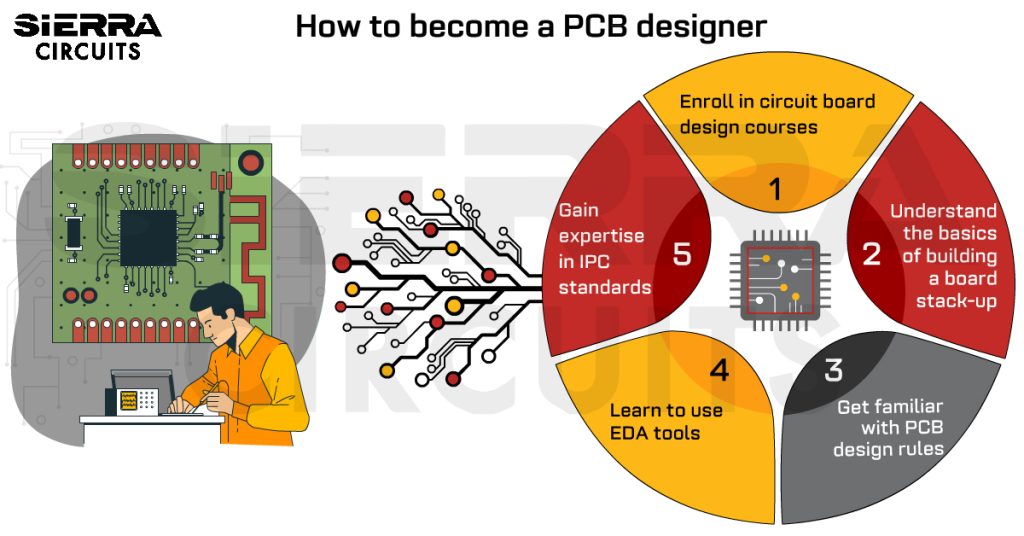
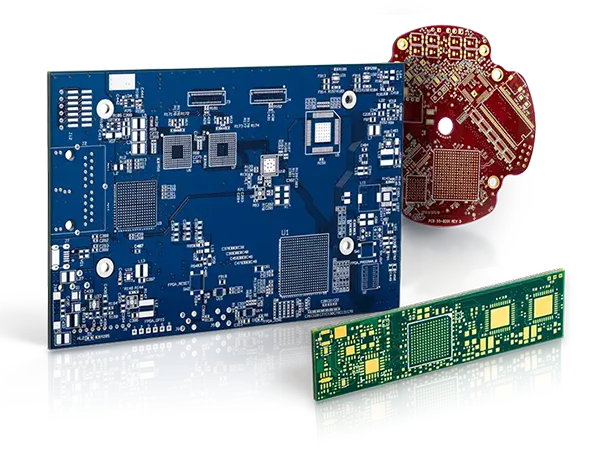
Contents

On-demand webinar
How Good is My Shield? An Introduction to Transfer Impedance and Shielding Effectiveness
by Karen Burnham
Electronic Anatomy or PCBs in Electronics
Doctors are like that supreme power on earth. They have been here, treating people since almost stone age. But as time advanced, the criticality, as well as success rates of the treatment of doctors, also advanced. Their skills and dedication have remained constant throughout time. So what changed? If you look closely, the secret behind this exponentially rising success rate in healthcare is the technological advancements. Medical technological advancement is vaguely synonymous to electronic machines in here which in turn is completely dependent on PCBs. Therefore, we can say the advancements in the medical sciences in recent times are a result of collective efforts. The knowledge of both human anatomy and electronic anatomy (read PCB), drives the healthcare situation.
The Unsung Heroes: Medical PCBs
After we explored the military-grade PCBs in a previous article, here also we are going to take-on something equally important and sensitive. Yes! Medical PCBs, the PCBs that are used in medical instruments. The present era is experiencing the best in class medical facilities. People are recovering easily from problems that were once dreaded to be the messenger of death. Patients are defying death and are recovering from those deadly diseases or attacks, or any almost any sort of organ failures. Doctors, the real-life heroes, no doubt deserve applauds but our unsung PCB heroes too equally deserve a loud shout-out. The responsibility that designers and manufacturers of medical electronic devices have on their shoulders day-in and day-out is unimaginable. They are responsible for the proper functioning of the medical appliances which in turn is responsible for a life.
Healthcare ranges from small wearable devices that promote wellness to full-body imaging systems that analyze the health of internal organs. Patient care, research or training medical professionals all are part of medical PCB world. Today we find PCBs in everything from cardiovascular medical specification PCBs to medical imaging systems. Be it pacemakers, defibrillators and heart monitors or, MRIs, CT scans, ultrasonic equipment. You will also find PCBs for medical PCBs in body temperature monitors, blood glucose monitors and electrical muscle stimulation equipment.
Read our blog medtech pcb design considerations with IPC and UL standards to learn more.
Technology and Healthcare
Ideally speaking, any new technological advancement is going to drastically affect people and their way to be. Yet, when new technology is entering the medical domain, the stakes are always a bit higher. Granted, not every medical device is going to be life-or-death, but they should still be considered carefully. PCBs fit in the unique constraints of medical devices, hence, are highly specialized.
In most medical applications, a small package is required to meet the size compulsion for an implant or emergency room monitor. They require a greater number of connections on lesser real estate. Moreover, power usage in a medical device should be as less as possible. Longer battery life in handheld and other battery-powered devices is very critical. Another very important aspect of medical PCBs is signal integrity. You will want to trust that any connected device is capable of transmitting the correct signal within expected timeframes. Basically, medical PCBs are miniaturization on a large scale. Therefore, medical PCBs tend to be high density interconnects or HDI PCBs.
But when discussing electronics miniaturization in terms of innovative medical technology, how small is small?
In the PCB market, a recent term is getting viral, “micro-miniaturization”. It is meant for the delivery of pharmaceuticals via implanted electronics. This technology has a fancy name “electroceuticals.” IC chips that are small enough to “swim” throughout the bloodstream and are powered wirelessly from outside the body is happening for real. These designs are supposed to regulate the delivery of medications by internally sensing what the body needs. Electroceuticals are beyond the average electronic medical device. At least as for now.
Medical PCBs: Rules and Standards
I am sure at this point, the thing bothering you is the thought of the electronics used in health centers. The accuracy of a medical device can determine whether a condition is properly diagnosed, therefore, the proper treatment can be provided. The precision of operation or whether the desired prognosis is delivered. The fact that the life of your loved one is dependent on such vivid factors is sure to make you giddy. But do not worry as all the medical electronics are bound to follow quite a few safety and quality standards.
To ensure medical devices meet their critical system performance mandate, the International Electrotechnical Commission (IEC), International Standards Organization (ISO), US Food and Drug Administration (FDA), Federal Communications Commission (FCC) and other agencies publish medical device regulations and standards to which companies building and supplying medical equipment must comply.
Also read, Case Study: PCBs for Healthcare and Life Science Industry
In the case of medical electronics design and development, the most widely used textbook is over 14 years old! Why?
To a great extent, these standards are geopolitically controlled. Each country having its own governing bodies and standards. Having said that, abiding by standards like IEC and ISO mostly covers all the countries. Think of it in this way, the fact that medical devices are so different and used in many different areas. Which means there will be specific regulations for specific devices, depending on how and where they are used. Due to this continuously evolving nature of regulations, creating a complete listing is virtually impossible. Electronic innovations in medical technology have been accelerating in a way that, lists get outdated before it hits the press. UL certification is also very important because it is issued by a safety organization that sets industry standards for manufacturing products.
However, to supply organizations with medical devices and equipment, there are a few key regulations you should be aware of.
-
ISO 13485 Medical Devices: Quality Management Systems (QMS) – Standards for Regulatory Purposes
It defines the specific requirements to the medical devices industry with respect to the entire sample space of QMS. ISO 13485 is designed for organizations involved in the design, production, installation, and servicing of medical devices. It also enables internal and external parties, such as certification bodies, with their auditing processes.
A medical device is a product, to be more precise an instrument, machine, implant or in vitro reagent, that is intended for assistance in the diagnosis, prevention, and treatment of medical conditions.
-
ISO 14971 Medical Devices: Risk Management Application to Medical Devices
This standard specifies a process for a manufacturer to identify the menace associated with medical devices, including in vitro diagnostic (IVD) medical devices. It estimates and evaluates the associated risks, how to control these risks, and monitoring the effectiveness of the controls.
ISO 14971:2007 standards stand applicable throughout all stages of the life-cycle of a medical device.
-
IEC 60601: Medical Electrical Equipment
IEC 60601 is a clan of standards whose latitude covers the safety, essential performance and electromagnetic compatibility of medical electrical equipment and Systems. The clan comprises over 70 separate standards.
Medical electrical equipment is defined in the here as electrical equipment having an applied part or transferring energy to or from the patient or detecting such energy transfer to or from the patient.
The “Part 1” standard, IEC 60601-1 covers basic safety and essential performance for all medical electrical equipment. For instance, electromagnetic compatibility (IEC 60601-1-2) or protection for diagnostic use of X-rays (IEC 60601-1-3) fall under this part.
The “Part 2” standard, IEC 60601-2 is all about “particular” standards cover requirements for specific product groups or specific measurements built into products. For e.g. MR scanners (IEC 60601-2-33) or electroencephalograms (IEC 60601-2-26). Collateral and particular standards may have their own revisions which differ from general standard.
-
US FDA 21 CFR Part 807
Center for Devices and Radiological Health (CDRH)is a subset of FDA which is responsible for regulating firms who manufacture, repackage, relabel, and/or import medical devices sold in the United States. Additionally, CDRH regulates radiation-emitting electronic products (medical and non-medical) such as lasers, x-ray systems, ultrasound equipment, microwave ovens, and color televisions.
-
Medical Device Listing – 21CFR Part 807
This listing under US FDA calls for all sort of manufacturers, distributors, repackagers, relabelers, specification developers, reprocessors single-use devices, importers and exporters to register their organization with the FDA.
-
FCC Title 47
The FCC’s rules and regulations are mentioned in Title 47 of the Code of Federal Regulations (CFR).
This defines the electromagnetic interference (EMI) standards for electronic equipment certifications. It certifies any electronic equipment that contains or is an RF device. An RF device can be defined as a device that is capable of emitting energy in the frequency range of 9kHz-3000GHz
P.S.: Your medical device may be subjected to other specific regulations and can include additional specific standards.
Apart from the standards, there are some key factors that regulate the success of medical circuit boards.
- The most significant point is the safety of the device.
- Precision is an essential factor, as we mentioned earlier as well the signal integrity, higher number of interconnects, lead-free component placements all these play a major role.
- Reliability and predictability are equally important as precision. It is only important that the device functions repetitively for the same situations, conditions or stimuli but does so over the lifetime of the device. To ensure this not only must your design be sound, but your CM must also use high-quality components and materials.
- The ease of use or usability is another significant contributor. To maximize your chances of successful medical device development, you must maintain the delicate balance between capability and usability.
After talking so much of regulations and compliance and standards, let’s look into some of the interesting and intriguing advancements in medical PCBs.
We have already mentioned about electroceuticals in our article, now we shall talk about sensitizing human senses. Technology since a long time has been playing a significant role in sight and hearing aids. But there are other senses that need our attention too, complying to that researches and studies are being done to form a skin with sensing capabilities. It is a stretchable, rugged “skin” that is essentially a printed circuit with thousands of transistors. Though it’s still in its infancy, we can expect Standford to come up with their results soon.
Ingestible sensors for digestion diagnosis can diagnose different gases in the stomach. The device is about the size of a large oblong, vitamin capsule, and is simply swallowed by the patient. This is believed to be serving a diagnostic tool for many stomach related issues.
The End Note by Sierra Circuits
The history of evolution has taught us that sometimes revolution is the only way and is almost inevitable. The same is with the medical industry, the evolution in this field has been gradual yet steep. In today’s information entangled life of ours, an assumption is never an option. Therefore, diagnosis on the basis of just symptoms can be misleading sometimes, proper test equipment makes it a matter of assurance. Medical PCBs have almost eliminated what we call misjudgment of a medical situation. It has transformed the way we lived and died as well. This humanitarian leap has been possible due to dedicated doctors and people related to health-care industry and along with that all those PCB designers, manufacturers, assemblers, suppliers, distributors, etc. who also have been working relentlessly on making our lives better. We work towards having a perfect world with all sort of health amenities.